|
XeF2蝕刻是各向同性的XeF2氣相矽蝕刻系統。用於去除Si作為犧牲層,XeF2去除是用於MEMS器件最好的釋放製程之一。XeF2除了蝕刻Si也可以刻蝕Ta,W,Mo等其他能被SF6等離子體蝕刻的材料
XeF2熔點低,25°
C時蒸汽壓為
4Torr,對矽具有很好的蝕刻作用,對Al
、SiO2 、SiNx、光阻劑等具有很好刻蝕選擇比。蝕刻速率
較快,可以達到1um/min
。
XeF2與水可以反應,生成氫氟酸
。氣態蝕刻,沒有液體張力問題的干擾
適合高深寬比製程
XeF2與矽反應分為以下步驟:
非游離態的XeF2吸附在矽表面
XeF2分解,產生F基
F基與矽反應,生成SiF4
反應產物分子SiF4形成氣相產物
不發生反應的殘餘氣體(分解產生的Xe)從矽表面上揮發。對於CHF3、CF4以及CCl4等蝕刻氣體,同樣情況下自發發生反應的機率很小,因此需要應用等離子體技術來產生反應基F,而如果採用XeF2,在沒有等離子體放電的情況下就可以得到高速率的矽蝕刻。蝕刻反應可用下述反應式描述:2XeF2+Si→2Xe(g)↑+SiF4(g)↑
在蝕刻反應中SiF4是主要的反應產物,但是也有少量的其他反應副產物,包括SiF3、SiF2、SiF以及Si2F6。這些反應產物有時會沉積在蝕刻表面,影響刻蝕的均勻性,與其他矽刻蝕方法相比採用XeF2蝕刻矽具有一定的優越性。
基片可選擇性大:由於刻蝕完全是一個化學反應過程,由於殘餘氣體都可以排出,所以基片尺寸可以減小
橫向刻蝕:由於是各向同性的蝕刻,可以對掩模圖形進行掏空
成本較低:蝕刻設備相對簡單,對環境的要求較低,不需要外加電源對氣體進行電離。
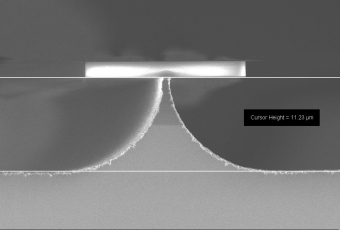
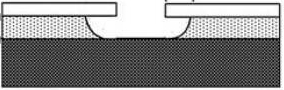
蝕刻氣體:Xenon
difluoride
(XeF2)對Al、SiO2、Si3N4、Au、TiNi、acrylic
及光阻有極高的選擇性,故而適於CMOS元件的後處
.
Interhalogen (XeF2、ClF2、BrF2):具有氣體蝕刻的優點但不會產生粗糙的表面。蝕刻速率Al、Cu、Au、Ni=1000:1,SiO2:Si=3000:1,Si3N4=400
– 800:1,AZ4400、AZ1518=1000:1
Xenon difluoride is
a very powerful fluorinating agent, but it is one of the most
stable xenon compounds. Like most covalent inorganic fluorides
it is moisture sensitive. It decomposes on contact with light
or water vapour. Xenon difluoride is a dense, white
crystalline solid. It has a nauseating odour but low vapor
pressure. It has a strong characteristic IR doublet at 550
cm−1 and 556 cm−1.
Material Specification Sheet Xenon Difluoride
XeF2
SPECIFICATION
|
Material |
Specification (wt ppm) |
Typical analysis (wt ppm) |
|
XeF2 |
99.999 % |
99.9994 % |
|
Al |
|
0.63 |
|
Ca |
|
0.30 |
|
Co |
|
0.16 |
|
Cr |
|
0.39 |
|
Cu |
|
0.09 |
|
Fe |
|
1.73 |
|
K |
|
0.16 |
|
Li |
|
0.02 |
|
Mg |
|
0.32 |
|
Mn |
|
0.04 |
|
Mo |
|
0.21 |
|
Na |
|
0.15 |
|
Ni |
|
1.55 |
|
Total metals |
<
10 |
5.75 |
Product is free of any XeF4 contamination
Supplied in PTFE bottles, gas cylinders or customer supplied
containers
Physical properties
Physical form
Colourless/white crystals
Molecular weight
169.29
Vapour pressure
approximately 4 Torr (0.5 kPa) at 25° C
Melting point
129° C
Boiling point
114° C
Stable molecule up to 500° C
Density 4.32 g/ml
Packing density 2 g/ml
Soluble in water (25 g/L at 0° C) and hydrolyses to form HF
Odor
Ozone like
Synthesis,
Properties and Chemistry of Xenon(II) Fluoride
A New Full-Dry Processing Method for MEMS
Rapid
Sacrificial Germanium Etching Using Xenon Difluoride
Influence of Reaction with XeF2 on
Surface Adhesion of Al and Al2O3 Surfaces
Determination of etching parameters for
pulsed XeF2 etching of silicon using chamber pressure data
Design, fabrication and characterization
of monolithic embedded parylenemicrochannels in silicon
substrate
Silicon Etching in XeF2 Environment
XeF2 Etching of Epitaxial Nb2N for
Lift-off or Micromachining of III-N Materials and Devices
Manipulating Etch Selectivities in XeF₂
Vapour Etching
Application of Dual-Doped TMAH Silicon
Etchant in the Fabrication of a Micromachined Aluminum Flexing
Beam Actuator
Etch Rates for Micromachining Processing
Dry silicon etching for MEMS
Etch Overview for Microsystems
If you don't find what you're looking for,
Contact Us.
We may have a suitable product that's not listed, or we may be
able to develop a material to fit your specific needs.
Tel : (02)2217-3442 / Fax : (02)2704-4070
|