Parylene Conformal Coating Specifications & Properties
I. Introduction
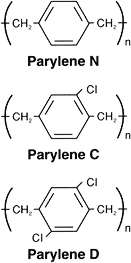
Parylene is the generic name for members of a unique polymer series. The basic member of the series, called Parylene N, is poly-para-xylylene, a completely linear, highly crystalline material.
 Parylene C, the second commercially
available member of the series, is produced from the same monomer modified
only by the substitution of a chlorine atom for one of the aromatic
hydrogens. The structures are shown in Figure 1. Parylene N,C & D
Chemical Structures (right).
Parylene C, the second commercially
available member of the series, is produced from the same monomer modified
only by the substitution of a chlorine atom for one of the aromatic
hydrogens. The structures are shown in Figure 1. Parylene N,C & D
Chemical Structures (right).
Parylene D, the third member of the series, is produced from the same monomer modified by the substitution of the chlorine atom for two of the aromatic hydrogens. Parylene D is similar in properties to Parylene C with the added ability to withstand higher use temperatures.
Parylene N is a primary dielectric, exhibiting a very low dissipation factor, high dielectric strength, and a dielectric constant invariant with frequency. This form has the highest penetrating power of all the Parylenes. Parylene C has a useful combination of electrical and physical properties plus a very low permeability to moisture and other corrosive gases. Along with its ability to provide a true pinhole free conformal insulation, Parylene C is the material of choice for coating critical electronic assemblies.
Due to the uniqueness of the vapor phase deposition, the Parylene polymers can be formed as structurally continuous films from as thin as a fraction of a micrometer to as thick as several mils.
II. The Deposition Process
The Parylene polymers are deposited from the vapor phase
by a process which in some respects resembles vacuum metallizing. Unlike
vacuum metallization, however, which is conducted at pressures of
10-5 torr or below, the Parylenes are formed at around 0.1
torr. Under these conditions the mean free path of the gas molecules in
the deposition chamber is in the order of 0.1 cm. Therefore, unlike vacuum
metallizing, the deposition is not line of sight, and all sides of an
object to be encapsulated are uniformly impinged by the gaseous monomer.
This is responsible for the truly conformal nature of coating.
 Click here for Figure 2 |
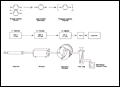
The process consists of three distinct steps as outlined in Figure 2 for Parylene N. The first step is vaporization of the solid dimer at approximately 150° C. The second step is the quantitative cleavage (pyrolysis) of the dimer at the two methylene-methylene bonds at about 680° C to yield the stable monomeric diradical, para-xylylene. Finally, the monomer enters the room temperature deposition chamber where it simultaneously adsorbs and polymerizes on the substrate. No liquid phase has ever been isolated and the substrate temperature never rises more than a few degrees above ambient. A necessary fourth component in this system is the mechanical vacuum pump and associated protective traps.
The equipment can be modified to meet the requirements or configuration of the items to be coated. Present coating chambers vary in size from 23 cm in diameter and 31 cm long to a 85 cm high hemi-cylindrical unit. One particular configuration, 45 cm in diameter and 60 cm high, was designed specifically for circuit boards and can accommodate up to two hundred 12 cm wide by 16 cm long boards. The process is inherently simple and can be conducted with a minimum of supervision.
Compared with vacuum metallizing, deposition rates are fast, especially for Parylene C, which is normally deposited at about 0.2 mils/hr. The deposition rate of Parylene N is somewhat slower. Kinetic studies have established that this rate is directly proportional to the square of the monomer concentration and inversely proportional to the absolute temperature.
III. Properties
 Click here for Table 1 |
The electrical, mechanical, thermal, barrier, optical, and other properties of Parylene N, C and D are discussed below. These properties are compared to those reported for other conformal coating materials such as epoxides, silicones, and urethanes.
A. Electrical Properties
The electrical properties of
Parylene N, C, and D are shown in
Table
1.
1. Thin Film Dielectric Properties
 Click here for Figure 3 |
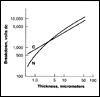
One of the features of the Parylenes is that they can be formed in extremely thin layers. The breakdown dc voltages of both Parylene C and N films have been determined as a function of polymer thickness, a plot of which is shown in Figure 3. Parylene C is superior to N in this respect for films under 5 micrometers (0.0002 in). These data show that both Parylenes have excellent dielectric withstanding voltages even below the 1 micrometer thickness range, and the voltage breakdown per unit thickness generally increases with decreasing thickness.
2. Circuit Board Insulation Resistance
A critical test
of the protection afforded by the Parylene coating is to coat circuit
board test patterns (as described in MIL-I-46058C) and subject them to
insulation resistance measurements during a temperature-humidity cycle
(MIL-STD-202, methods 106 and 302).
 Click here for Table 2 |
In brief, this test consists of ten cycles (one cycle per day), with each cycle consisting of seven steps. The seven steps range from low temperature, low humidity (25° C, 50% RH) to more severe conditions (65° C, 90% RH). Resistance readings are taken initially and at the 65° C, 90% RH step for each cycle of the ten day test.
Results are shown in Table 2 for Parylene C coating thicknesses from 0.002 in to 0.0001 in. It is interesting to note that even for the very thin coatings (0.0001 in), the insulation resistance values are about one order of magnitude above the prescribed specification.
B. Physical and Mechanical Properties
Physical and mechanical
properties of the Parylenes are summarized in
Table 3.
Because of their high molecular weight (~500,000) and because the melting
temperatures and crystallinity are high, the Parylenes cannot be formed by
conventional methods such as extrusion or molding. Solubility in organic
or other media, except at temperatures above 175° C, is so low that they
cannot be formed by casting.
 Click here for Table 3 |
Impact resistance is high when the Parylene polymers are supported on test panels. Gardner falling ball impact tests on 0.001 in thick Parylene C coated steel "Q" panels are in the 250 in-lb range.
Wear index values (measured on a Taber Abraser machine using CS-17 "Calibrase" wheel with 1000 gram weight) were 22.5 for Parylene C and 8.8 for Parylene N. By comparison, polytetrafluoroethylene (Teflon®) is 8.4, high impact polyvinylchloride is 24.4, epoxide is 41.9 and urethane is 59.5.
C. Thermal, Cryogenic, and Vacuum Properties
1. Thermal
Based on an Arrhenius extrapolation of test
data, Parylene C is expected to survive continuous exposure to air at 100°
C for ten years (100,000 hours). In oxygen free atmospheres, or in the
vacuum of space, the Parylenes are expected to serve similarly on
continuous exposure to 220° C . In all cases, higher temperature shortens
use life. If the requirements for your application are near or exceed this
time-temperature-atmospheric conditions envelope, it is recommended that
you test the complete structure under conditions more closely resembling
the actual conditions of intended use.
 Click here for Table 4 |
Parylene may be annealed to increase cut-through resistance, increase hardness, and improve abrasion resistance. This is the result of a density and crystallinity increase.
General thermal properties are summarized in Table 4.
2. Cryogenic
Steel panels coated with Parylene C and
chilled in liquid nitrogen at -160° C withstood impacts of more than 100
in-lb in a modified Gardner falling ball impact test. This compares with
values of about 250 in-lb at room temperature.
Unsupported 0.002 in films of Parylene C can be flexed 180° six times at -165° C before failure occurs. Comparable films of polyethylene, polyethylene terephthalate, and polytetrafluoroethylene fail at three, two, and one flexes, respectively.
Neither electrical nor physical properties are affected by cycling from 2° K (-271° C) to room temperature.
3. Vacuum Stability
Vacuum tests conducted at the jet
Propulsion Laboratory show that total weight loss at 121° F and 10-5 torr
were 0.12% for Parylene C and 0.30% for Parylene N. Volatile collectible,
condensable material values were less than 0.01% (the limit of sensitivity
of the test) for both polymers.
D. Barrier Properties and Chemical Resistance
 Click here for Table 5 |
1. Barrier
The barrier properties of the Parylenes are
given in
Table
5. The moisture vapor transmission rates are again compared with
those of other conformal coating materials. The rate for Parylene C is
superior to almost all polymeric materials.
Moisture vapor permeability values have been measured at thicknesses below 0.1 micrometer. Normalized to equivalent thickness, the values are the same for all thicknesses.
2. Chemical Resistance
The Parylenes resist room
temperature chemical attack and are insoluble in all organic solvents up
to 150° C. Parylene C can be dissolved in chloro-napthalene at 175° C, and
Parylene N is soluble at the solvent's boiling point (265° C). Both
polymers are resistant to permeation by most solvents. They also are
unaffected by stress-cracking agents such as Hostepal®, Igepal®, an lemon
oil.
 Click here for Figure 4 |
E. Optical Properties, Radiation, and Weathering Resistance
1. Optical Properties
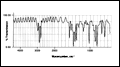
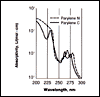
Parylene exhibits very little absorption in the visible region and is, therefore, transparent and colorless. Below about 280 nm both Parylene N and C absorb strongly, as shown in Figure 4.
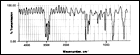
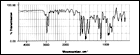
The infrared spectra for 1 mil films are shown in Figure 5, Figure 6, and Figure 7.
2. Radiation and Weathering Resistance
Parylene N, C
and D films show a high degree of resistance to degradation by gamma rays
in vacuum. Tensile and electrical properties were unchanged after 100 Mr
dosage at a dose rate of 1.6 Mr/h. Exposure in air leads to rapid
embrittlement.
Although stable indoors, the Parylenes are not recommended for long term use outdoors when exposed to direct sunlight.
IV. Adhesion
Good to excellent adhesion of Parylene to a wide variety
of substrates can be achieved by the simple treatment with a dilute
solution of an organic silane prior to Parylene coating. Two silanes,
vinyl trichlorosilane in either xylene, isopropyl alcohol, or Freon® and
gammamethacryloxypropyltrimethoxysilane in a methanol-water solvent have
been successfully used. The techniques were developed primarily for
electronic components and assemblies and do not affect any of the
substrate or coating properties. Silanes can also be vapor phase applied
if non-liquid application is preferable. Generalized procedures for using
these silanes are available upon request.
V. Product Safety
Specialty Coating Systems has compiled the information
contained herein from what it believes are authoritative sources and
believes that it is accurate and factual as of the date printed. It is
offered solely as a convenience to its customers and is intended only as a
guide concerning the products mentioned, Since the user's product
formulation, specific use application, and conditions of use are beyond
Specialty Coating Systems control, Specialty Coating Systems makes no
warranty or representation regarding the results that may be obtained by
the user. It shall be the responsibility of the user to determine the
suitability of any products mentioned for the user's specific application.
We urge you to review, prior to use, the Material Safety Data Sheets for
any Specialty Coating Systems products mentioned herein. These documents
are available from your Specialty Coating Systems sales representative.
This information is not to be taken as a warranty or representation for
which Specialty Coating Systems assumes legal responsibility nor as
permission to practice any patented invention without a license.
VI. Applications
A. Conformal Coating of Printed Circuit Boards for
Protection Against Moisture and Corrosive Environments
 The ability to deposit Parylene as a truly
conformal, thin, continuous, uniform adherent coating permits its
application as a protective PCB coating.
The ability to deposit Parylene as a truly
conformal, thin, continuous, uniform adherent coating permits its
application as a protective PCB coating.
A number of printed circuit assemblies and conductor patterns have been cross-sectioned and polished after coating with Parylene. Figure 8. Scale drawing of 0.024 in wire coated with 0.001 in of Parylene - 50X (above-right) is a representation of a photomicrograph taken of a 0.024 in wire on a phenolic laminate, drawn to scale at 50X magnification.
 The Parylene C coating was 0.001 in thick. The
Parylene polymer penetrated and coated uniformly even in the 0.002 in gap
between the wire and board.
The Parylene C coating was 0.001 in thick. The
Parylene polymer penetrated and coated uniformly even in the 0.002 in gap
between the wire and board.
Figure 9. Cross section of Conductor Pattern on Glass-Epoxy Laminate - 50X (left) shows a photomicrograph of a conductor pattern on an epoxy glass laminate coated with 0.002 in. of Parylene C. Replication of the step-edge is particularly impressive.
B. Surface Passivation and Environmental Protection of Semiconductor Devices
Parylene at thicknesses from 0.I micrometer to 0.002 in, has been found useful in this application. Again, the truly conformal nature of the coating allows bonded wires and evaporated conductor patterns to be coated at the same thickness as the silicon or other semiconductor surfaces. Parylene at thicknesses down to at least 0.4 micrometer is pinhole free as shown by HF and chlorine etch techniques.
Thorough evaluation proves that Parylene does not alter the electronic parameters of devices. Furthermore, when Parylene coated devices, integrated and hybrid circuits, are encapsulated by transfer molded epoxide compounds their performance is equivalent to hermetically sealed units in accelerated aging tests (pressure cooker).
C. Conformal Coating of Biomedical Devices for Environmental Protection and Electrical Insulation Requirements
Thin Parylene films can be deposited on virtually any biomedical substrate. Parylene coated devices are stable, exhibit little or no change in response characteristics, and are electrically and chemically isolated from the body.
D. Parylene has found acceptance in a myriad of industrial, aerospace, Chemical, automotive, consumer, pharmaceutical, and defense applications
Copyright and Disclaimerc © Xuan Bao Hao Corp. 隱私保護及安全聲明 | 智慧財產權 請使用IE 4.0以上版本之瀏覽器